Soil Testing and Analysis: What to Expect in the Report
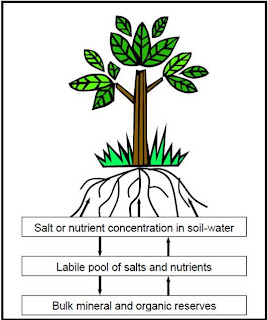 Agricultural laboratories usually analyze and report soil salinity and fertility levels in the same report. Saturation percentage, electrical conductivity, sodium, calcium, magnesium, chloride, bicarbonate and carbonate, sulfate, and boron are all part of salinity. Indices such as the Sodium Adsorption Ratio (SAR) and Exchangeable Sodium Percentage (ESP) are calculated from these basic measurements of soil salinity and included in the report. Soil testing for salinity is designed to diagnose osmotic effects, specific ion toxicities, and infiltration problems. When the salinity (electrical conductivity) of the soil-water surrounding the root exceeds the tolerance of a salt sensitive crop like walnut, the gradient between the solute concentration in the root cells and the soil-water around the root lessens, reducing water availability to trees. Trees influenced by osmotic effects will not grow as vigorously. Specific ion toxicity involves the accumulation of sodium, chloride, or boron in soils to high enough levels that the risk of these elements accumulating to toxic levels in leaf tissue of trees increases. Symptoms of ion toxicity may include death of leaf tissue along the margins or in the interveinal areas of leaves. Soils that develop slow water infiltration and permeability rates are sometimes related to low levels of electrical conductivity and calcium, and high levels of sodium or magnesium.Fertility focuses on essential plant nutrients, which is evaluated based upon soil pH and proper quantities of nitrogen, phosphorus, potassium, zinc, iron, manganese, copper, and molybdenum in the soil to promote walnut tree growth and fruit development. While calcium, magnesium, boron, and chloride are important to diagnose salinity, they are also of interest from the standpoint of fertility in terms of deficiency, sufficiency, and balances.Salts and nutrients exist in soils as three forms (see schematic) and this is reflected in soil test reports. Bulk minerals and organic reserves release salts and nutrients very slowly and contribute only minor to the quantity or intensity of soil salinity or fertility affecting crops, so commonly used soil tests do not measure bulk minerals and organic reserves. Bulk minerals and organic reserves do play an important role in buffering soil salinity and fertility.Labile forms of salts and nutrients are unstable and constantly seeking equilibrium with the salts and nutrients in the soil-water and to a lesser extent with the bulk mineral and organic reserves. It is widely accepted for laboratories to measure labile forms of nitrate-nitrogen, orthophosphates, and potassium in soils. They can be recognized on the soil test report by measurement units in “milligrams per killogram soil (mg/kg)”. Many, but not all, laboratories measure and report salts in the labile form. If measured, they can be recognized in a report by descriptive terms such as “exchangeable”, “extractable”, “cation exchange capacity” or reporting units in “milliequivalents per 100 grams of soil (meq/100 gm)”. Some laboratories prefer to only measure and report salts in the saturated paste extract rather than exchangeable forms, unless specifically requested by the customer. Measuring exchangeable cations and the cation exchange capacity can be challenging because research has shown that the best available laboratory methods of extracting cations adsorbed to the clay particles also dissolve lime and gypsum in the soil and as a result tend to overestimate the cation exchange capacity and exchangeable calcium.Nearly all laboratories measure salinity (sodium, calcium, magnesium, chloride, bicarbonate and carbonate, sulfate, and boron) in a saturated paste extract. The extract represents the soil-water at a very high soil moisture content along with the dissolved salts and nutrients that are readily available to the tree. Decades of research on crop tolerance to salinity have been based upon correlations between crop development, yield and salinity levels in the extract. The extract is acquired for testing by following specific procedures to make a saturated soil paste or mud and then vacuum suctioning the water from the paste. The water content of the saturated paste (saturation percentage) is measured and reported to understand the dilution effect of the saturated soil and to enable the test results to be related to lower soil moisture levels that typically occur in orchard soils. Salinity measurements of the saturated soil extract are often labeled on reports as “water-soluble” and the reporting units are “milliequivalents per liter”. The soil pH reported is commonly measured in the saturated paste prior to vacuum extracting the soil-water from the saturated paste. Nutrients such as nitrate-nitrogen, phosphorus, and potassium may not be reported in the water-soluble forms because determinations of these nutrients in labile forms are more meaningful to evaluate soil fertility.For further information about soil testing and analysis and to acquire specific guidelines for interpreting soil salinity and fertility results in walnut, some suggested references include: 1) Chapter 19, Nutrients in the Soil and Chapter 26, Nutritional Deficiencies and Toxicities- Diagnosing and Correcting Imbalances in UC Publication 3364 Almond Production Manual, 1996; and 2) UC Publication 3375, Agricultural Salinity and Drainage, 2006. The North American Proficiency Testing Program website (http://www.naptprogram.org), which operates as an activity of the Soil Science Society of America, seeks to assist soil, plant, and water testing laboratories in their performance through inter-laboratory sample exchanges and statistical evaluation of laboratory results. The website provides information about participating laboratories in California and throughout the United States. Technical information is also available on laboratory methods specifically used on soils in the western United States including California conditions.
Agricultural laboratories usually analyze and report soil salinity and fertility levels in the same report. Saturation percentage, electrical conductivity, sodium, calcium, magnesium, chloride, bicarbonate and carbonate, sulfate, and boron are all part of salinity. Indices such as the Sodium Adsorption Ratio (SAR) and Exchangeable Sodium Percentage (ESP) are calculated from these basic measurements of soil salinity and included in the report. Soil testing for salinity is designed to diagnose osmotic effects, specific ion toxicities, and infiltration problems. When the salinity (electrical conductivity) of the soil-water surrounding the root exceeds the tolerance of a salt sensitive crop like walnut, the gradient between the solute concentration in the root cells and the soil-water around the root lessens, reducing water availability to trees. Trees influenced by osmotic effects will not grow as vigorously. Specific ion toxicity involves the accumulation of sodium, chloride, or boron in soils to high enough levels that the risk of these elements accumulating to toxic levels in leaf tissue of trees increases. Symptoms of ion toxicity may include death of leaf tissue along the margins or in the interveinal areas of leaves. Soils that develop slow water infiltration and permeability rates are sometimes related to low levels of electrical conductivity and calcium, and high levels of sodium or magnesium.Fertility focuses on essential plant nutrients, which is evaluated based upon soil pH and proper quantities of nitrogen, phosphorus, potassium, zinc, iron, manganese, copper, and molybdenum in the soil to promote walnut tree growth and fruit development. While calcium, magnesium, boron, and chloride are important to diagnose salinity, they are also of interest from the standpoint of fertility in terms of deficiency, sufficiency, and balances.Salts and nutrients exist in soils as three forms (see schematic) and this is reflected in soil test reports. Bulk minerals and organic reserves release salts and nutrients very slowly and contribute only minor to the quantity or intensity of soil salinity or fertility affecting crops, so commonly used soil tests do not measure bulk minerals and organic reserves. Bulk minerals and organic reserves do play an important role in buffering soil salinity and fertility.Labile forms of salts and nutrients are unstable and constantly seeking equilibrium with the salts and nutrients in the soil-water and to a lesser extent with the bulk mineral and organic reserves. It is widely accepted for laboratories to measure labile forms of nitrate-nitrogen, orthophosphates, and potassium in soils. They can be recognized on the soil test report by measurement units in “milligrams per killogram soil (mg/kg)”. Many, but not all, laboratories measure and report salts in the labile form. If measured, they can be recognized in a report by descriptive terms such as “exchangeable”, “extractable”, “cation exchange capacity” or reporting units in “milliequivalents per 100 grams of soil (meq/100 gm)”. Some laboratories prefer to only measure and report salts in the saturated paste extract rather than exchangeable forms, unless specifically requested by the customer. Measuring exchangeable cations and the cation exchange capacity can be challenging because research has shown that the best available laboratory methods of extracting cations adsorbed to the clay particles also dissolve lime and gypsum in the soil and as a result tend to overestimate the cation exchange capacity and exchangeable calcium.Nearly all laboratories measure salinity (sodium, calcium, magnesium, chloride, bicarbonate and carbonate, sulfate, and boron) in a saturated paste extract. The extract represents the soil-water at a very high soil moisture content along with the dissolved salts and nutrients that are readily available to the tree. Decades of research on crop tolerance to salinity have been based upon correlations between crop development, yield and salinity levels in the extract. The extract is acquired for testing by following specific procedures to make a saturated soil paste or mud and then vacuum suctioning the water from the paste. The water content of the saturated paste (saturation percentage) is measured and reported to understand the dilution effect of the saturated soil and to enable the test results to be related to lower soil moisture levels that typically occur in orchard soils. Salinity measurements of the saturated soil extract are often labeled on reports as “water-soluble” and the reporting units are “milliequivalents per liter”. The soil pH reported is commonly measured in the saturated paste prior to vacuum extracting the soil-water from the saturated paste. Nutrients such as nitrate-nitrogen, phosphorus, and potassium may not be reported in the water-soluble forms because determinations of these nutrients in labile forms are more meaningful to evaluate soil fertility.For further information about soil testing and analysis and to acquire specific guidelines for interpreting soil salinity and fertility results in walnut, some suggested references include: 1) Chapter 19, Nutrients in the Soil and Chapter 26, Nutritional Deficiencies and Toxicities- Diagnosing and Correcting Imbalances in UC Publication 3364 Almond Production Manual, 1996; and 2) UC Publication 3375, Agricultural Salinity and Drainage, 2006. The North American Proficiency Testing Program website (http://www.naptprogram.org), which operates as an activity of the Soil Science Society of America, seeks to assist soil, plant, and water testing laboratories in their performance through inter-laboratory sample exchanges and statistical evaluation of laboratory results. The website provides information about participating laboratories in California and throughout the United States. Technical information is also available on laboratory methods specifically used on soils in the western United States including California conditions.
